In a regulated lab, compliance isn’t just about checking boxes, it’s about building systems that naturally prevent errors, protect data integrity, and ensure reproducibility. Whether your lab aligns with GLP, GCP, CLIA, ISO 15189, or FDA expectations, the principles behind compliance are the same: maintain control, consistency, and traceability across every sample, process, and data point.
At Computype, we help laboratories achieve these goals through thoughtful identification strategies that combine durability, automation, and intelligent design. Below are practical ways that improvements in labeling and sample identification can help your lab not only meet compliance standards but build confidence in every result.
Build Traceability from the Ground Up with Barcoding
Barcoding is one of the simplest but most powerful compliance tools available. By assigning a unique barcode to every tube, slide, or plate, you establish a digital chain of custody that supports GLP, CLIA, and FDA documentation requirements.
Barcodes make it easy to:
- Track sample movement across instruments and personnel
- Link physical specimens to electronic records (LIMS or ERP systems)
- Reduce transcription errors that could compromise data integrity
- Input sequence data quickly via scanning
For example, one Computype partner lab reduced manual entry errors substantially after implementing barcode-based sample tracking and automated scanners at key workflow points.
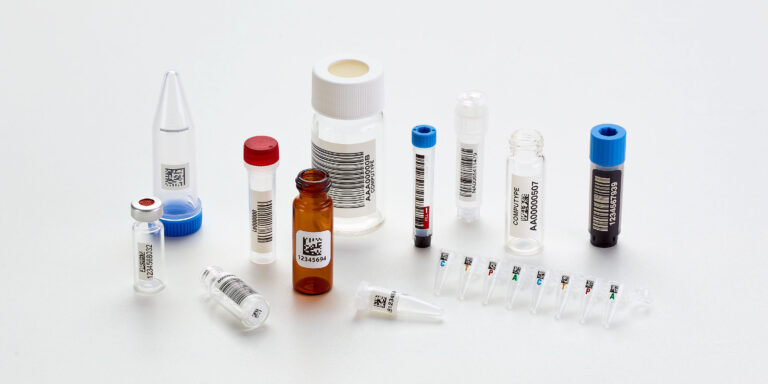
Maintain Data Integrity with Sequence and Lot Management
Compliance frameworks consistently emphasize traceable, reproducible workflows. A structured sequence management system, where every label series, pre-marked lot, and print batch is documented, helps labs demonstrate complete process control.
Pre-serialized labels or labware make it possible to:
- Ensure no duplication or skipped identifiers
- Audit label histories during investigations or inspections
- Easily align labeling sequences with your internal recordkeeping systems
This approach eliminates the “manual numbering” risks that inspectors often flag and supports traceable, auditable sample management from creation to disposal.

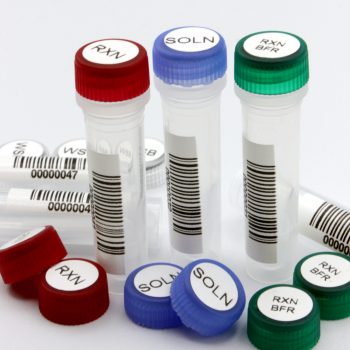
Use Color Coding to Prevent Mix-Ups and Improve Visual Control
Color coding does not just look organized; it is a practical safeguard. Assigning color to represent departments, sample types, or protocols provides an additional layer of verification for both staff and auditors.
A color-coded labeling system:
- Makes deviations or misplaced samples immediately visible
- Simplifies cross-training and visual identification in busy labs
- Helps demonstrate proactive risk management in quality reviews
For instance, implementing color-coded caps and labels for different study phases or patient groups can prevent costly sample crossover errors in GCP and CLIA environments.
Choose the Right Materials for the Right Conditions
Using preferred, validated label materials is essential for maintaining sample identity through all processing steps. Compliance frameworks emphasize that labeling must remain legible and affixed under all anticipated conditions, whether that is cryogenic storage, autoclaving, staining, or solvent exposure.
This is especially relevant in applications where FDA-approved or compliant adhesive constructions are necessary. Selecting adhesives and face stocks that are verified for these environments helps ensure both regulatory alignment and sample security.
Computype’s material recommendations ensure:
- Labels resist fading, smearing, or detachment through entire workflows
- Materials are chemically compatible with your reagents and storage conditions
- The same label construction can be specified and reproduced consistently for audits
By standardizing materials, your lab can demonstrate due diligence and control. These practices are key principles of ISO 15189, GLP and FDA documentation practices.
Improve Consistency Through Pre-Marked Labware
Pre-marked labware provides a controlled, ready-to-use foundation for compliance. Tubes and vials that arrive already labeled or pre-marked with unique identifiers eliminate variability from manual labeling and ensure consistency across lots, shifts, and sites.
This helps labs:
- Reduce handling and labeling time
- Ensure identifier permanence even under harsh conditions
- Simplify training by removing subjective, manual labeling steps
- Ensure necessary data is present and properly positioned for simplified reading or scanning downstream
For labs managing thousands of samples weekly, pre-marked labware is a simple way to improve both throughput and audit readiness.

Standardize Templates and Workflows for Repeatability
Labeling consistency directly supports documentation and process control requirements. Using standardized label templates, printer settings, and approval workflows ensures that every label printed meets the same specification, regardless of who prints it.
This practice supports compliance by:
- Guaranteeing that critical data (ID, date, operator, etc.) is always present and correctly formatted
- Preventing unapproved label edits or untracked design changes
- Making your labeling process fully reproducible and audit-traceable
When your labeling system mirrors your SOPs and quality manual, compliance follows naturally.
Partnering Toward Confidence and Control
Compliance is built on strong systems, disciplined processes, and well-trained teams, but the right tools can make those efforts far more effective. By incorporating barcoding and safeguarded, effective labeling practices, laboratories can strengthen the accuracy and consistency of their workflows.
At Computype, our role is to aid in designing reliable identification and labeling solutions that integrate seamlessly into your quality systems, reduce variability, and provide confidence that your samples and records will stand up to review. Computype provides on-site evaluation and support to identify bottlenecks, assess label performance in real workflows, and recommend practical improvements related to labeling and barcode reliability.
Whether you’re preparing for an audit, updating SOPs, or scaling to new testing capabilities, Computype partners with you to build labeling strategies that support compliance goals, improve traceability, and bring greater control to daily operations.
Ready to strengthen your lab’s compliance foundation?
Connect with our labeling experts to explore labeling and automation strategies that align with your workflows and quality standards.
Connect with our labelling experts today
Blog article form
"*" indicates required fields
