Behind every scientific advancement are thousands of biological samples all carefully stored and preserved for medical research. The integrity of these samples depends not only on how they’re stored but also on how each vial is labeled and tracked. However, this research could be threatened when older labels fail or when they need to be updated to be compatible with a new or upgraded automation system. As technology continues to advance, expectations around data management and reporting are becoming more important. Reliable labeling plays a crucial role in helping labs keep better records and meet compliance expectations.
Many labs are transitioning to automated workflows to improve throughput and accuracy. This also increases the need for consistent documentation because regulators look for strong traceability. Clear identification through barcoding plays a significant role in keeping records consistent. Automation also builds the consistency and traceability that many labs later rely on as they move toward higher compliance standards.
For the vials in cryogenic storage, the challenge is finding a way to bring legacy samples up to modern labeling standards without compromising their contents through freeze-thaw cycles. Some older vials may have handwritten labels or linear barcodes that aren’t compatible with modern equipment or LIMS, ELN, and other information management systems. This becomes especially important for labs that manage patient linked samples. Re-labeling these vials without thawing or compromising sample integrity becomes a challenging task.
This is where Computype’s expertise in identification and tracking helps bridge the gap. We can help barcode your samples to be compatible with automation systems, or to meet newly implemented regulatory standards. Our cryogenic labeling solutions provide a reliable, automation-ready solution to re-label samples with durable 2D barcodes that can be placed anywhere, on any labware. This means legacy samples remain frozen, traceable, and ready for the next stage of scientific progress.
Challenges of Re-Labeling Legacy Vials
Many vials stored in cryogenic freezers are labeled with linear barcodes or may not even have a barcode but simply handwritten markings. These labeling methods have become outdated and are not compatible with automated workflows. They also do not support today’s information or asset management systems, which can hold organizations back when working toward compliance or preparing to move into automated platforms.
When each vial carries valuable information such as collection date, sample volume, and processing details, it must be accurately recorded to preserve sample integrity. Without a consistent barcode to store this information, valuable samples can become difficult to locate, trace, or validate.
Relabeling those samples isn’t simple. Thawing vials risks damaging sensitive biological material, while frost and condensation make label adhesion difficult on frozen vials. Labs need a labeling solution that adheres securely to frozen vials without thawing or disrupting the data associated with each sample.
Relabeling Samples with 2D Barcodes
Modern labs are investing in automation systems to improve accuracy and efficiency. For legacy vials that have a handwritten label, it’s recommended to add a barcode to your vial. By adding a barcode, your vial contents will be added and stored to your LIMS or other inventory management system for quick and easy tracking. This allows you to track the location of every sample in your freezer and liquid nitrogen dewars, reducing the time it takes to retrieve samples and minimizing sample thawing.
Adding a 2D barcode to your legacy samples is one of the most effective ways to modernize your biorepository. A 2D barcode captures more information in a smaller space, making it ideal for integration with automation platforms and LIMS. Unlike linear barcodes, a 2D barcode can be scanned instantly from the top or bottom of your vial, allowing automation equipment to read entire trays at once instead of handling each vial individually. The result is faster processing with fewer manual touchpoints, preserving sample integrity and improving overall workflow efficiency.
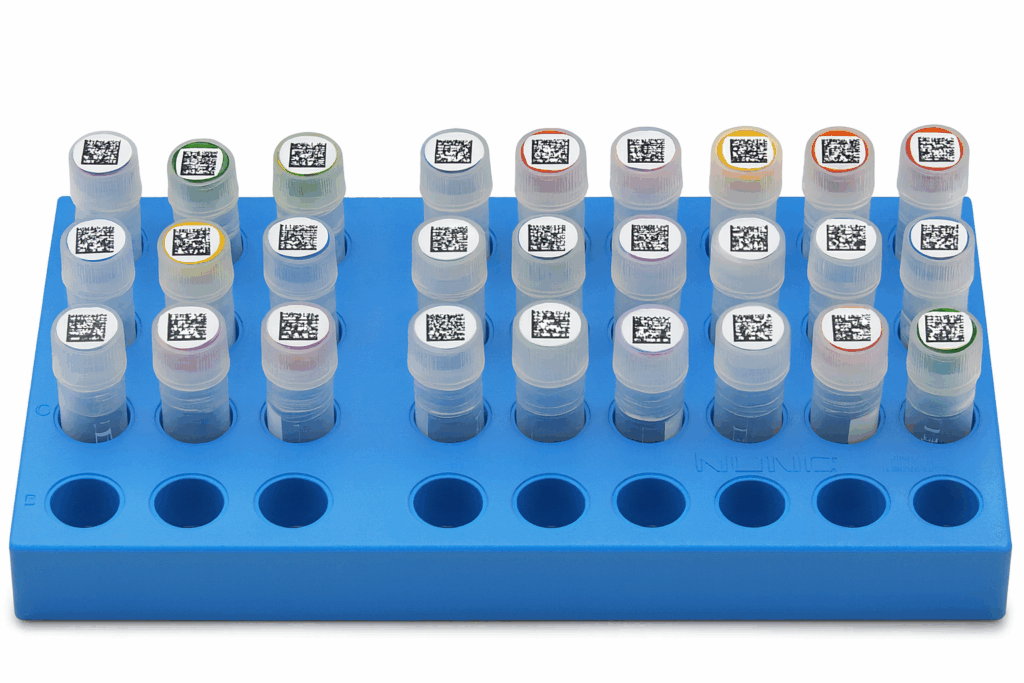
While off-the-shelf systems may limit you to specific tubes or vial formats, our approach provides complete flexibility. We can create a system where your samples can be stored in well-plates or any type of container including flip cap tubes, bottles, 15- and 50-mL conical tubes, cryo vials and more. This allows your team to upgrade identification without changing existing workflows or performing lengthy validation studies. Clear labeling also supports better data hygiene, which has become an essential part of how modern labs manage information and maintain accurate records.
Cryogenic Labeling Solutions for Legacy Vials
To meet these needs, we offer two cryogenic labeling solutions designed specifically for re-labeling legacy vials in frozen storage while maintaining compatibility with automated systems.
Puck Labels
Pucks are small circular inserts that fit into the top of cryogenic vial or tube closures, often referred to by manufacturers as color coders. Our cryogenic puck labels are applied to the insert with a customizable 2D barcode, enabling fast, accurate labeling while samples remain frozen.

Bottom Labels
For labs whose automation systems are configured to scan from below, bottom labels provide another reliable option for identifying frozen vials. These labels feature a compact 2D barcode at the center, paired with customizable wraparound arms that extend up the sides of the vial for added surface adhesion and stability. This design keeps the barcode securely in place, even in ultra-low temperature environments.

Both label options are engineered to adhere to frozen glass or plastic surfaces as cold as -80°C and withstand liquid nitrogen temperatures down to -196°C. Each includes a unique 2D data matrix barcode that integrates seamlessly with your LIMS, allowing every vial to be tracked within SBS-format racks. Together, they provide flexible options for safely re-labeling legacy samples and aligning your inventory with modern standards. For labs upgrading to automation, these solutions restore order and traceability to frozen collections. Standardized labeling also makes it easier for labs to scale their operations without losing track of historical samples.
Barcode Sequence Management for Better Accuracy
To make barcode tracking even more reliable, we also offer barcode sequence management services that save time and improve accuracy. Our proprietary software eliminates duplicate sequences, maintaining complete integrity across your entire sample inventory. We support all major barcode symbologies, including QR codes, DataMatrix, Code 128, and UPC, with expert design to ensure flawless scans. With expert barcode sequence management, your lab can trust that every identifier stays unique, traceable, and fully compatible with your automation setup.
These labeling and sequence management tools also support data migration into validated LIMS, ELN, and biobank databases. This is now best practice for labs preparing for future regulatory requirements or working toward GMP or FDA readiness. Legacy samples without consistent IDs or audit trails represent a growing compliance risk and bringing them up to modern standards protects long term research value.
Ready to Modernize Your Legacy Samples?
Labs that have adopted our puck or bottom labels report faster workflows, improved inventory accuracy, and more efficient retrieval of archived samples. By eliminating manual scanning and reducing sample handling, they save hours each week while maintaining the integrity of every frozen sample.
The path to automation doesn’t have to leave legacy samples behind. With the right labeling solution, your archived inventory becomes part of a modern, reliable system built for long term scientific growth and regulatory confidence. Re-labeling frozen vials doesn’t need to be complicated or risky. With pre-printed 2D barcoded labels and our Labware Prep™ Services, where we can do the labeling for you, we can help your team transition smoothly into automation.
Contact us today to get started and see how we can help re-label your legacy vials in cryogenic storage, so they remain accurate, traceable, and fully compatible with your automation workflow and future compliance needs.
Connect with our labelling experts today
Blog article form
"*" indicates required fields
