Securely label blood bags and tubes to meet regulatory standards. Our labels are designed for accurate tracking and identification, even in cold storage conditions.
Explore our specialized blood product labels, including blank kits and pre-labeled options. Ensure your blood products are managed with precision and compliance.


Blood labeling requires precision, durability, and full regulatory compliance. From blood bags and tubing to test samples and cryo units, every label must perform reliably across collection, processing, and storage. We offer flexible options to support your workflow—from blank label kits to fully printed and applied solutions.
Shop blank labels with matching ribbons
Order labels fully printed to spec
Add printers or label applicators as needed
Get help choosing the right label construction
Label sets for whole blood and red cell units, including base labels, integrated quadrant labels, and DIN compliance—engineered to adhere securely to curved, plasticized bag surfaces.
Labels for collection and testing tubes, matched to DIN sets or used independently. Designed for tight diameters and capable of withstanding centrifugation, refrigeration, and chemical exposure.
Flat bag and frozen-unit labels built for storage at -80°C and below. Materials resist lifting, cracking, and fading to ensure barcodes remain scannable through thaw and transfer.

Layouts support DIN, product codes, and quadrant labels for full compliance and traceability across donation and processing workflows.
FDA 21 CFR 175.105–compliant adhesives, including piggyback options, ensure a secure bond to curved, low-energy bag materials.
Label designs meet broader regulatory expectations, including handling instructions, language requirements, and transfusion-ready formatting.
See how decades of expertise shape our industry-leading DIN label sets. This video highlights our role in advancing traceability, our commitment to ISBT 128 compliance, and the precision formatting that ensures every label supports reliable blood identification—from collection to transfusion, across bags, tubes, and segment tracking.
Follows the ISBT 128 standard, including a 13-character DIN with facility ID and check digit.
Applied to bags, tubes, and segments to link all products and test samples from a single donation.
Enables global traceability and ensures each blood unit remains uniquely identifiable at every stage.
Base labels identify the blood component using ISBT 128 product codes, expiration dates, and anticoagulant type.
Quadrant or segment labels carry matching DINs for traceability across tubing segments and downstream testing.
Label constructions suit curved surfaces, cold storage, and multi-zone layouts for donation and processing data.
Support infectious disease testing, blood typing, and compatibility workflows using matched identifier labels.
Fit securely on small-diameter tubes while remaining legible and scannable after processing and storage.
Prevent mix-ups by printing DIN-synced labels at the time of collection or via traceable batch sets.
Ensure accurate component labeling for flat plasma bags, cryo containers, or specialty platelet packaging.
Display product codes, expiration dates, and storage requirements using compact ISBT 128-compliant layouts.
Enable secure labeling even in frozen or high-humidity environments with appropriate adhesives and materials.
Highlight conditions such as “irradiated,” “CMV negative,” or “autologous only” for downstream handling.
Use color codes, icons, or preprinted warnings to support at-a-glance recognition by clinical staff.
Pair with core labels to maintain ISBT compliance while providing added clarity at the point of use.
Explore label formats in action—request a sample to see how each type performs on blood bags, tubes, and components.
Explore specialized features made possible by our label converting and finishing expertise—from double-adhesive builds to automation-ready formats—engineered specifically for blood bags, tubes, and traceability workflows.

Durability isn’t optional in blood labeling—it’s essential. Labels must survive constant handling, temperature shifts, chemical exposure, and equipment contact without lifting, smudging, or failing. From blood bags in coolers to tubes in centrifuges, every component of your label construction is engineered to stay intact and scannable throughout its full lifecycle.
Resists alcohol wipes and bleach cleaning
Withstands cold storage and thaw cycles
Maintains adhesion on curved plastic surfaces
Preserves barcode legibility through handling and transport
Piggyback labels enable quadrant or segment labels to be cleanly transferred to tubing for traceability, using double-permanent adhesives engineered for curved surfaces and FDA 21 CFR 175.105 compliance.
4″x4″ blood bag labels with a notched corner ensure consistent DIN placement and visual hierarchy, supporting ISBT 128 compliance while allowing modular pairing of pre-printed and variable data label sets.
Precision-cut labels engineered for consistent placement on blood collection tubes, compatible with high-speed print-and-apply systems and designed to withstand storage, centrifugation, and post-collection handling workflows.
Face stocks for blood labeling must withstand curved plastic surfaces, temperature fluctuations, and chemical exposure. We utilize a variety of synthetic films—including specially treated polypropylene and other engineered plastics—designed to maintain stability and print quality under stress. These materials resist shrinkage, winging, and delamination when exposed to common medical cleaning agents, UV light, and cold storage. Their dimensional stability ensures scan reliability for barcodes throughout the lifecycle of the blood product. Certain face stocks are specifically formulated for high-speed label applicators, minimizing static and improving registration accuracy. Selection is driven by compatibility with adhesives, label format (e.g., full bag vs. segment), and intended print method. Material thicknesses and finishes can be customized to support legibility, scannability, and durability. All face stocks used in blood label constructions are vetted for performance on plasticized PVC and similar medical-grade substrates.
Adhesion to blood bags presents unique challenges due to the use of plasticizers in flexible PVC films, which can interfere with long-term bond integrity. Our adhesives are engineered to resist plasticizer migration and maintain adhesion across a wide temperature range, from refrigerated storage to ambient use. Permanent acrylic systems—including both emulsion and solvent formulations—are selected for their high initial tack and long-term shear resistance. We offer specialized constructions such as double-layer (piggyback) adhesives for label transfer and multi-stage applications, frequently used for DIN and quadrant labels. All blood bag adhesives are compliant with FDA 21 CFR 175.105, ensuring suitability for indirect contact with blood containers. Performance testing includes rub resistance, temperature cycling, and compatibility with automated label applicators. We tailor bond strength and dwell time based on your application method, surface texture, and intended storage conditions.
Surface coatings and laminates play a critical role in preserving print integrity and enhancing resistance to environmental exposure. For thermal transfer and digital print compatibility, we offer topcoats that crosslink with resin ribbons or toner systems to lock in printed images, making them resistant to alcohols, disinfectants, and water. In high-abrasion environments, optional overlaminates—available in clear, matte, or UV-filtered variants—can be applied to shield variable data and barcode fields without affecting readability. Laminates may be applied edge-to-edge or in selective zones depending on label format. Coating formulations are selected to minimize static buildup and support high-speed application. Label constructions for cryogenic plasma storage or long-term freezer use can incorporate coatings with added flexibility and cold-crack resistance. All coatings and laminates are tested for compatibility with the specific face stock and adhesive used in the label structure.
Barcode and identifier permanence is essential in blood labeling. We support a wide range of resin and resin-enhanced thermal transfer ribbons engineered for chemical and abrasion resistance, especially when printed onto coated synthetic face stocks. These ribbons produce high-density, sharp barcodes suitable for ISBT 128 and other symbologies. For digital workflows, our label materials are compatible with toner-based and inkjet systems, and can be pre-coated to enhance toner anchorage or ink receptivity. Inks used for pre-printed static content (e.g., facility ID, label borders) are tested for bleed resistance and long-term legibility on curved plastic bags. Print durability is validated using rub and wipe tests simulating alcohol and bleach exposure. When print-on-demand is required, ribbon and label pairings are optimized together to maintain scannability in cold or variable storage environments.
Blood label geometries must accommodate a wide range of products and packaging formats, from full-size bags to tubing segments and sample tubes. We offer customizable label sizes to fit common blood bag layouts, including standard 4″ × 4″, 4″ x 2″, and 2″ × 2″ formats, DIN-notch designs, and integrated quadrant/segment labels. Small-format labels for test tubes and segment strips are precision-cut to remain secure on narrow diameters and smooth surfaces. Our die-cutting capabilities support complex shapes, peel-off sections, and multiple label components per liner to enable modular labeling strategies. Adhesive-free zones, perforations, and tamper indicators can also be incorporated. Dimensional tolerances are tightly controlled to support high-speed applicators and ensure label placement accuracy in both manual and automated workflows. Label sets can be delivered in rolls, fanfolds, or sheeted formats as needed.
Blood labels must perform reliably across a wide range of physical, chemical, and environmental conditions. We engineer durability into every layer of the construction—from face stock to adhesive to topcoat—to ensure resistance to abrasion, moisture, cleaning agents, and temperature extremes. Labels for blood bags are designed to stay intact on flexible PVC surfaces during handling, transport, and refrigeration, while segment labels maintain print integrity even on narrow tubing. Materials are tested for resistance to alcohol wipes, bleach, water immersion, and blood contamination, with constructions available to withstand temperatures from deep freeze (-80°C) to ambient processing environments. For high-volume workflows, surface durability is critical to ensure that barcodes remain scannable and text remains legible throughout the unit’s lifecycle. In-house testing includes rub tests, chemical exposure, and temperature cycling to validate label performance under real-world conditions.
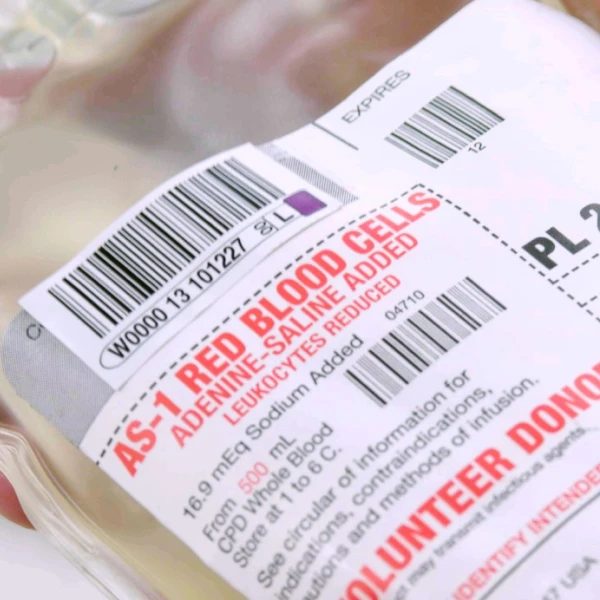
Shop blank label sets built for thermal transfer printing—each paired with compatible ribbons and designed for durability, compliance, and reliable barcode performance across blood bags, tubes, and segment formats.






Have questions? We’re here to help.
Contact us to connect with a specialist who understands your industry and can provide the right solutions for your business. Let’s start a conversation.











© Computype 2024
© Computype 2024
Take advantage of our volume discounts for bulk orders. Reach out to us for a personalized quote tailored to your needs.
"*" indicates required fields