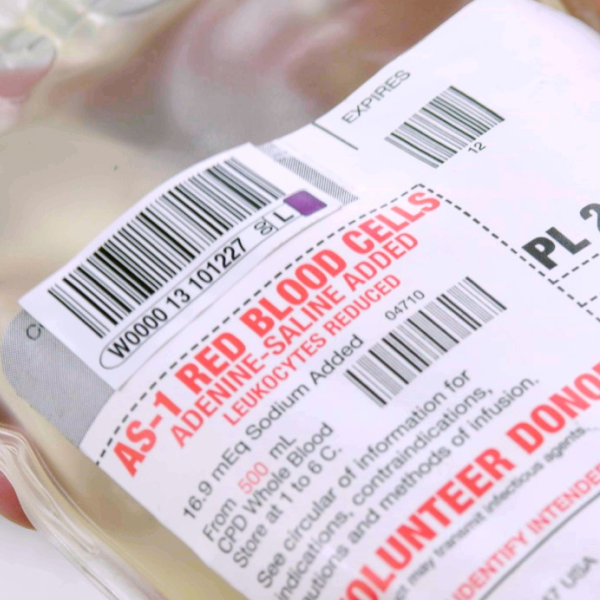
Securely label blood bags and tubes to meet regulatory standards. Our labels are designed for accurate tracking and identification, even in cold storage conditions.
Explore our specialized blood product labels, including blank kits and pre-labeled options. Ensure your blood products are managed with precision and compliance.


Our blood labels are engineered to meet the rigorous demands of blood collection, processing, and transfusion. Designed for durability and clarity, these labels ensure that all critical information remains legible throughout various stages of handling, from donation to transfusion.
Quadrant labels divide the labeling area into four sections, each containing essential information for traceability and compliance with ISBT 128 standards. These sections typically include data such as the ABO/RhD blood group, Donation Identification Number (DIN), product code, and expiration date. This modular system allows flexibility in applying quadrants separately, ensuring all critical information is correctly aligned on the final label.
Base labels are applied to blood bags and cell therapy containers before processing begins, providing essential manufacturer information such as the container lot number and catalog number. These labels must meet specific size and placement requirements to ensure they do not interfere with final labeling, which is added after processing. Base labels serve as a foundation, ensuring traceability from the manufacturing stage through to the end use.
DIN labels carry the Donation Identification Number, a 13-character code that ensures each donation is uniquely identifiable worldwide. The DIN includes a facility identifier, year of donation, and a sequence number, along with flag characters for process control. DIN labels are critical for maintaining traceability across various stages of handling and processing, ensuring accurate identification throughout the product’s lifecycle
Follow standard ISBT 128 label sizes to ensure that blood products are labeled consistently and accurately. ISBT 128 is an internationally recognized standard for labeling blood, cells, and other human-origin medical products, designed to maintain traceability and safety across global healthcare systems.


DIN labels ensure that each blood donation is uniquely traceable throughout its lifecycle. The 13-character Donation Identification Number (DIN) includes a facility code, year code, and a sequence number, creating a globally unique identifier. This system is critical for traceability and product safety in compliance with ISBT 128.
13-character identifier to ensure global traceability.
The DIN also contains flag characters for process control and a check character for data accuracy. This identifier is encoded as a machine-readable barcode, allowing for accurate scanning at every stage.
DIN labels streamline handling and tracking at every critical processing stage, maintaining accuracy and full traceability throughout. This system significantly reduces risks by ensuring precise tracking from the collection of the donation to its final use in transfusions. Blood banks and healthcare facilities worldwide rely on DIN labels for highly efficient and secure donation management processes.
Our blood product labels are crafted with advanced adhesives designed to withstand the rigorous demands of the blood collection and transfusion processes. These labels are also compliant with FDA CFR regulations, ensuring that they meet the highest standards for safety and performance in medical environments.
Double (piggyback) adhesives simplify pre-printed application.
They allow a preprinted face stock to be precisely applied to a secondary adhesive layer after removing a temporary liner, ensuring accurate placement and secure adhesion during the final labeling process.
Our blood labels are designed to enhance both the accuracy and efficiency of labeling processes in critical environments. By utilizing advanced adhesives, including double (piggyback) systems, these labels ensure secure and precise placement, even in challenging conditions. The materials are carefully chosen to conform to various surfaces while maintaining clear, legible information.





Maximize the efficiency and accuracy of your blood labeling operations with our dedicated hardware and software solutions. These tools are tailored to simplify label replication, enhance traceability, and ensure compliance with stringent regulatory standards.

DIN replicators offer a comprehensive solution for quickly and accurately replicating Donation Identification Number (DIN) labels. This is ideal for blood banks and transfusion services that need to update or duplicate DIN labels with minimal effort—ensuring every label is consistent and compliant.











© Computype 2024
© Computype 2024
Take advantage of our volume discounts for bulk orders. Reach out to us for a personalized quote tailored to your needs.
"*" indicates required fields