In the pharmaceutical, biotech, and healthcare industries, where precise temperature control is critical for vaccines, biologics, and personalized medicine, cold chain labeling ensures product integrity and regulatory compliance. These sensitive materials must be accurately tracked and properly labeled throughout their journey to ensure they remain viable and effective. Cold chain environments introduce extreme conditions that can degrade standard labels, making it critical to use specialized cold chain labeling solutions.
Durable, cold-resistant labels ensure that pharmaceuticals, biological reagents, and vaccines are clearly identified, traceable, and compliant with stringent regulatory standards. In this article, we’ll explore the importance of reliable cold chain labeling and how it ensures the accuracy and safety of pharmaceutical products.
The Role of Labeling in Cold Chain Pharmaceutical Management
Cold chain management is essential for maintaining the efficacy of temperature-sensitive pharmaceuticals. From manufacturing to distribution, every stage of the supply chain must be carefully managed to maintain consistent temperatures, and labeling plays a vital role in ensuring accurate tracking and regulatory compliance. Here’s why labeling is so critical in cold chain pharmaceutical management.
Ensuring Product Identification and Traceability
Accurate labeling ensures that pharmaceuticals, biologics, and vaccines are properly identified at every stage of their journey, from production and packaging to storage and distribution. Each container must be labeled with key information, including batch numbers, expiration dates, and storage conditions. Traceability is critical in the event of recalls or quality issues, as the ability to track individual batches back to their source ensures rapid corrective action.
Maintaining Label Integrity in Extreme Conditions
Pharmaceutical cold chain environments expose materials to extreme cold, often reaching -80°C (dry ice) or -196°C (liquid nitrogen) for biologics and gene therapies. Labels that cannot withstand these conditions may become brittle, peel off, or lose adhesion, compromising the identification and tracking of critical products. Cold-resistant labels are essential to maintaining label integrity, ensuring that information remains legible and secure throughout storage and transport.
Regulatory Compliance in Cold Chain Labeling
The pharmaceutical cold chain is one of the most heavily regulated supply chains due to the sensitivity and high value of its products. Regulatory bodies enforce strict guidelines to ensure the safety, efficacy, and traceability of pharmaceuticals. These regulations cover everything from manufacturing practices to labeling and serialization, ensuring that pharmaceutical products remain safe and effective throughout their lifecycle.
Pharmaceuticals, especially biologics and vaccines, are highly temperature-sensitive. Improper storage or labeling errors can result in degraded efficacy, financial losses, and potential patient harm. Regulatory agencies impose stringent rules to prevent errors in identification, tracking, and storage conditions. Several major regulatory frameworks set the standards for pharmaceutical cold chain labeling:
- FDA (21 CFR Part 211, Part 820) – Ensuring good manufacturing and labeling practices.
- USP 1079 – Best practices for good storage and distribution.
- EU Annex 1 (GMP for sterile medicinal products) – Regulatory standards for European markets.
- DSCSA & FMD (Serialization) – Track-and-trace requirements to prevent counterfeiting.
Beyond ensuring product efficacy, these regulations also serve to prevent counterfeiting and improve global traceability. Serialization and track-and-trace systems have become critical components of pharmaceutical supply chains, helping to eliminate fraud and ensure that drugs reach their intended destinations in the correct condition.
These regulations help standardize tracking, minimize errors, and ensure that critical medicines reach patients without compromise. To comply with these regulations, cold chain pharmaceutical labels must include:
- Product Name and Dosage – Clearly identifies the medication and its concentration.
- Batch/Lot Number – Ensures traceability in case of recalls.
- Expiration Date – Indicates the stability period under prescribed storage conditions.
- Storage Conditions – Details required temperatures, such as “Store between 2-8°C” or “Keep Frozen at -80°C.”
- Handling Instructions – Notes any special requirements, such as “Do Not Shake” or “Protect from Light.”
- Serialization and Barcode Information – Includes QR codes or 2D barcodes for automated scanning and verification.
These labeling and adhesive requirements ensure that pharmaceutical products remain in compliance with industry regulations and maintain their efficacy throughout the supply chain.
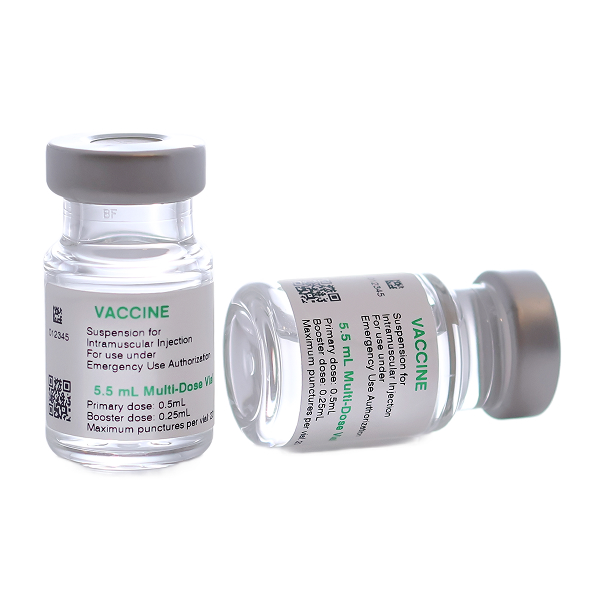
Key Features of Cold Chain Labels for Pharmaceuticals
Cold chain labels must be engineered to withstand the specific challenges posed by extreme temperatures, moisture, and frequent handling. Here are the key features of optimized pharmaceutical cold chain labels:
Cold-Resistant Materials and Adhesives
Cold chain labels must be made from materials that can endure extreme cold without becoming brittle, peeling, or losing adhesion. Labels made from polypropylene or polyester are ideal due to their ability to withstand low temperatures while maintaining clarity and adhesion. Additionally, cold-resistant adhesives ensure that labels stay securely attached even in freezing conditions, preventing misidentification or detachment.
Moisture and Chemical Resistance
Pharmaceutical cold chain shipments often experience moisture, condensation, and chemical exposure during transitions between temperatures. Labels that absorb moisture can smudge, fade, or lose adhesion, making them unreadable. Moisture-resistant labels prevent these issues by repelling water and ensuring that labels remain intact throughout the cold chain journey.
Durable Print and Barcode Readability
Pharmaceutical tracking relies on serialization, barcodes, and data matrices to ensure end-to-end traceability. Cold chain labels must maintain print durability, ensuring that text and barcodes remain readable even after exposure to extreme cold and moisture. Pre-barcoded labware improve workflow efficiency and reduce human error during scanning and verification.
Tamper-Evident and Security Features
Due to the risk of counterfeit drugs in the pharmaceutical supply chain, tamper-evident labels help ensure authenticity. Labels with destructible adhesives or holographic elements enhance security and compliance with global track-and-trace regulations like the DSCSA and FMD.
Long-Term Storage Durability
Many biologics and gene therapies require years-long storage in cryogenic environments, often undergoing multiple freeze-thaw cycles. Labels for these applications must maintain adhesion and readability over extended periods without degradation. Special coatings or laminates can further protect printed information from environmental wear, ensuring accurate identification for the entire storage duration.
Applications of Cold Chain Labels in Pharmaceutical Management
Cold chain labels are critical in various pharmaceutical applications, ensuring product identification and compliance at every stage. Here are some key areas where optimized labeling solutions make a significant impact:
Vaccine Storage and Distribution
Vaccines must be kept within strict temperature ranges to ensure efficacy. Cold-resistant labels ensure that vaccines are properly identified and tracked during distribution and storage, preventing handling errors and ensuring compliance with safety regulations. Moisture-resistant labels maintain legibility even when exposed to condensation during transit.
Biologics and Personalized Medicine
Monoclonal antibodies (mAbs), gene therapies, and autologous cell therapies require ultra-cold storage, sometimes reaching -196°C in liquid nitrogen. These high-value products demand cryogenic-grade labeling solutions that withstand extreme conditions without degrading or detaching.
Plasma and Blood Product Tracking
Biobanking and transfusion medicine rely on barcode-labeled blood and plasma samples. High-contrast barcode labels prevent misidentification, ensuring traceability from donation to patient administration while maintaining compliance with AABB and FDA regulations.
Cold Chain Logistics and Serialization
Pharmaceutical supply chains are heavily serialized to prevent counterfeiting and diversion. Cold chain labels integrate with track-and-trace systems, ensuring end-to-end visibility and compliance with global serialization mandates.

Operational Considerations for Cold Chain Labeling and Tracking
Supply Chain Continuity and Risk Mitigation
Pharmaceutical supply chains must ensure continuous operations to prevent costly disruptions. Standardizing label formats across global operations minimizes errors and ensures consistent identification across all distribution points. Labels should be designed to withstand potential supply chain delays and variations in handling conditions.
To mitigate risks, implementing multi-layered labeling—where primary and secondary labels serve as backups—ensures redundancy in case of label failure. Additionally, contingency planning for label recalls or misprints is crucial to avoid bottlenecks in distribution. Having pre-approved alternative labeling solutions allows for rapid responses in the event of unforeseen challenges.
Temperature-Sensitive Logistics Integration
Cold chain logistics involve multiple temperature transitions that can impact label performance. Ensuring compatibility between labels and temperature-monitoring devices such as data loggers helps maintain seamless tracking throughout the supply chain. Labels must also be able to withstand sudden shifts in temperature from storage to transport.
During last-mile delivery, exposure to ambient conditions can compromise label adhesion. Labels should be designed to maintain adhesion and readability despite condensation or exposure to fluctuating temperatures. Selecting labels that remain legible and intact under these conditions prevents errors in distribution and patient administration.
Standardization Across Partners and Vendors
Many pharmaceutical companies work with third-party logistics providers (3PLs), contract manufacturers, and global distributors. Defining label compliance standards ensures uniformity across all partners, reducing inconsistencies that can lead to regulatory delays.
Global standardization also involves ensuring that label designs meet country-specific regulations while maintaining compliance with overarching industry guidelines. Establishing automated workflows for serialization and data entry improves efficiency and reduces manual input errors that could impact inventory accuracy.
Automation and Scalability for High-Volume Operations
High-volume pharmaceutical distribution requires scalable labeling solutions that integrate seamlessly with automated inventory management systems. Labels should be optimized for high-speed application to support large-scale operations without slowing down production lines.
Automation also enables real-time tracking by utilizing machine-readable barcoding. This enhances logistics efficiency by reducing scanning errors and ensuring faster product verification at each checkpoint in the cold chain process.
Global Compliance for International Distribution
Pharmaceutical products are often shipped across multiple regulatory jurisdictions, each with its own labeling requirements. Labels must comply with regional regulations such as FDA (U.S.), EMA (Europe), and WHO (global vaccine distribution). In addition, multi-language labeling and country-specific serialization formats may be necessary to meet international guidelines and ensure seamless global distribution.
Cold chain environments present unique challenges for pharmaceutical labeling, especially when dealing with biologics, vaccines, and gene therapies. By selecting cold-resistant, moisture-resistant, and tamper-evident labels that are compliant with global pharmaceutical regulations, organizations can ensure that their products remain accurately identified and traceable throughout the cold chain.
Whether managing vaccine distribution, biologic storage, or serialized cold chain logistics, cold chain labels must offer durability, security, and clarity to ensure safety and compliance at every stage of the supply chain.
Continue Exploring Labeling Solutions
If you’re ready to take the next step, explore our most popular solutions:
Connect with our labelling experts today
Blog article form
"*" indicates required fields
